MicroSeal®-VP Low Endotoxin
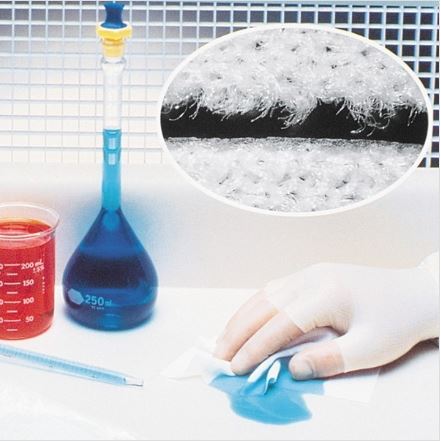
MicroSeal®-VP LE is a non-sterile sealed-edge polyester low endotoxin wipe that provides maximum protection when it comes to providing critically low particle generation for the most critical cleaning applications for the medical device, pharmaceutical, and other regulated industries where the smallest measure can make the biggest difference. Our patented surface treatment enables the MicroSeal®-VP LE to capture and retain particulate contamination and endotoxins well beyond the capabilities of a typical polyester sealed edge wipe. In addition, this wipe is also a high performer when it comes so chemical compatibility with many of the common disinfectant and cleaning solutions. In addition, all of our low endotoxin wipes are lot-to-lot tested to ensure they meet or exceed the requirements set by USP & FDA standards based on the maximum allowable thresholds for endotoxins across key regulated industries.
$0.00
ISO Class
3-5Construction
KnittedMaterial
PolyesterEdge
Ultrasonically SealedSKU
MSVP09098LE
Size
9″ x 9″ (23cm x 23cm)
# Per Pack
150
# Per Case
8
 View Product Information Sheet
View Product Information Sheet
Be the first to review “MicroSeal®-VP Low Endotoxin”
You must be logged in to post a review.









 View Product Information Sheet
View Product Information Sheet








Reviews
There are no reviews yet.